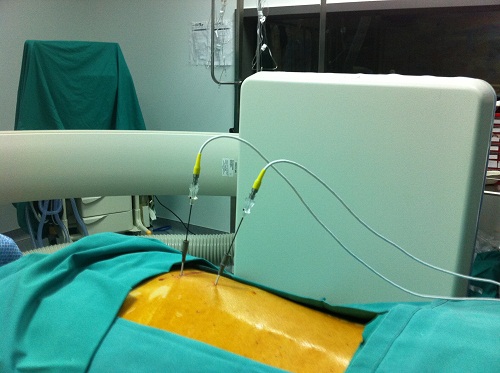
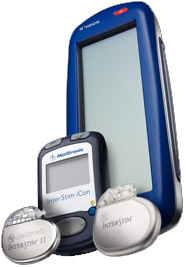
 The Journal Diseases of the Colon and Rectum recently published data from a multicenter, prospective trial showing that use of Medtronic’s InterStim Therapy via sacral nerve stimulation reduced incontinent episodes and improved quality of life in a majority of patients with chronic fecal incontinence at three years of follow up. Medgadget reported on the surgically implanted device when the FDA approved commercial distribution of IntreStim for chronic fecal incontinence earlier this year.
The Journal Diseases of the Colon and Rectum recently published data from a multicenter, prospective trial showing that use of Medtronic’s InterStim Therapy via sacral nerve stimulation reduced incontinent episodes and improved quality of life in a majority of patients with chronic fecal incontinence at three years of follow up. Medgadget reported on the surgically implanted device when the FDA approved commercial distribution of IntreStim for chronic fecal incontinence earlier this year.
The study indicated the long-term effectiveness and safety of InterStim use in patients with fecal incontinence of more than twice per week. 83 of the original 120 patients followed completed all or part of the 3-year follow-up assessment. At 3 years, 86% of subjects had greater than or equal to 50% reduction in incontinent episodes (mean 9.4 episodes/week reduced to 1.7 episodes/week). Furthermore 40% of patients had perfect continence. Overall quality of life improved for subjects. The most commonly reported adverse events were implant site pain, paresthesias, stimulation sensation changes, and infection.
The InterStim system is a surgically implanted neurostimulator device that delivers electric impulses via an implanted lead to the sacral nerve to modulate the anal sphincter, and hence incontinence. The device is similar in function to prior approved stimulation therapy for urinary incontinence.
Press release: Study Shows Medtronic InterStim® Therapy Improves Bowel Control and Related Quality of Life Issues at Three Years …
Abstract: Long-term Efficacy and Safety of Sacral Nerve Stimulation for Fecal Incontinence